 |
Cytokines
http://press2.nci.nih.gov/sciencebehind/immune/immune15.htm
Cytokines are diverse and potent chemical messengers secreted by the cells of the immune system—and
the chief tool of T cells.
Lymphocytes, including both T cells and B cells, secrete lymphokines, while monocytes and
macrophages secrete monokines.
Binding to specific receptors on target cells, cytokines recruit many other cells and substances
to the field of action. Cytokines encourage cell growth, promote cell activation, direct cellular traffic, and destroy target
cells—including cancer cells. Because they serve as a messenger between white cells, or leukocytes, many cytokines are
also known as interleukins
At least two types of lymphocytes are killer cells—cytotoxic T cells and natural killer
cells.
To attack, cytotoxic T cells need to recognize a specific antigen, whereas natural killer
or NK cells do not. Both types contain granules filled with potent chemicals, and both types kill on contact. The killer binds
to its target, aims its weapons, and delivers a burst of lethal chemicals.
Phagocytes and Granulocytes
Phagocytes are large white cells that can engulf and digest foreign invaders.
They include monocytes, which circulate in the blood, and macrophages, which are found in
tissues throughout the body, as well as neutrophils, cells that circulate in the blood but move into tissues where they are
needed. Macrophages are versatile cells; they act as scavengers, they secrete a wide variety of powerful chemicals, and they
play an essential role in activating T cells.
Neutrophils are not only phagocytes but also granulocytes: they contain granules filled with
potent chemicals. These chemicals, in addition to destroying microorganisms, play a key role in acute inflammatory reactions.
Other types of granulocytes are eosinophils and basophils. Mast cells are granule-containing cells in tissue.
Phagocytes in the Body :

Organs of the Immune System:

|
http://www.hon.ch/Library/Theme/Allergy/Glossary/leukotriene.html
Leukotrienes |
MeSH definition: A family of biologically active compounds derived
from arachidonic acid by oxidative metabolism through the 5-lipoxygenase pathway. They participate in host defense reactions
and pathophysiological conditions such as immediate hypersensitivity and inflammation. They have potent actions on many essential
organs and systems, including the cardiovascular, pulmonary, and central nervous system as well as the gastrointestinal tract
and the immune system.
| |
| Leukotriene B4 |
| MeSH definition: The major metabolite in neutrophil polymorphonuclear
leukocytes. It stimulates polymorphonuclear cell function (degranulation, formation of oxygen-centered free radicals, arachidonic
acid release, and metabolism). (From Dictionary of Prostaglandins and Related Compounds, | |

Leukotrienes and prostaglandins are derivatives of arachidonic acid (AA) an unsaturated fatty acid produced from membrane phospholipids. The principal pathways of arachidonic acid metabolism are :
- the 5-lipoxygenase pathway, which produces a collection of leukotrienes (LT) and
- the cyclooxygenase pathway, which yields a number of prostaglandins (PG) and thromboxanes
(Tx).
All three are synthesized by monocytes and macrophages. Mast cells and basophils generate a mixture of leukotrienes. The products of both pathways act in concert to cause inflammation with prostaglandins producing fever and pain. Aspirin, ibuprofen, and certain other nonsteroidal anti-inflammatory
drugs (NSAIDs) achieve their effects (fever and pain reduction) by blocking the activity of cyclooxygenase. [Discussion
leukotriene: http://cancerweb.ncl.ac.uk/cgi-bin/omd?leukotriene+a4
Chemical name: 6,8,10,14-Eicosatetraenoic acid, 5,12-dihydroxy-, (S-(R*,S*-(E,Z,E,Z)))-
Macrophages are involved at all stages of the immune response. First, as already outlined, they act as rapid
protective mechanism which can respond before T cell-mediated amplification has taken place
Monocytes lose their myeoloperoxidase activity during conversion to tissue macrophages
However, macrophages may acquire MPO from their environment by pinocytosis or from ingested neutrophils.
In this way, especially macrophages in inflammatory site with the intensive cell destruction, can gain myeloperoxidase (or
other peroxidase). Such peroxidase then participates in cytotoxic mechanisms of macrophages.
Macrophages are important producers of arachidonic acid and its metabolites. Upon phagocytosis macrophages
release up to 50% of their arachidonic acid from membranous esterified glycerol phospholipid. It is immediately metabolized
into different types of prostanoids. From them prostaglandins, especially PGE , and prostacyclin (PGI , and prostacyclin (PGI ) are characterized as pro-inflammatory agents: they induce vasodilatation, act synergeticly with complement component C5a
and LTB ) are characterized as pro-inflammatory agents: they induce vasodilatation, act synergeticly with complement component C5a
and LTB , mediate fever and myalgia response to IL-1, in the combination with bradykinin and histamine they contribute to erythema,
oedema, and pain induction. Tromboxan TXA , mediate fever and myalgia response to IL-1, in the combination with bradykinin and histamine they contribute to erythema,
oedema, and pain induction. Tromboxan TXA is considered as an inflammatory mediator; it facilitates platelet aggregation and triggers vasoconstriction. LTB is considered as an inflammatory mediator; it facilitates platelet aggregation and triggers vasoconstriction. LTB is the efficient chemoatractant substance. A mixture of LTC is the efficient chemoatractant substance. A mixture of LTC , LTD , LTD and LTE and LTE became known as slow-reacting substance of anaphylaxis (SRS-A). These leukotrienes are important mediators of bronchial asthma,
since they provoke long-term contractions of bronchial smooth muscl became known as slow-reacting substance of anaphylaxis (SRS-A). These leukotrienes are important mediators of bronchial asthma,
since they provoke long-term contractions of bronchial smooth muscl
A. Mast Cells [5] http://www.outlinemed.com/demo/allergy/4850.htm
See outline "Hypersensitivity Reactions"
- Mainly found in tissues, low numbers in blood of normal persons
- Usually found beneath epithelial surfaces and near blood vessels
- Long lived cells (weeks to months)
- Derived from CD34+ bone marrow progenitor cells
- Disbursed to tissue as precursor cells, probably related to basophils
- Mast cell precursors fully mature in specific tissues
- Development requires mast-cell growth factor, the ligand for the c-kit gene
- Preformed granules in cytoplasm, released on activation of cells
- Have immunoglobulin (Ig) Fc receptors, specific primarily for IgE
- Responsible for a variety of IgE dependent and some independent reactions
- Mast cell disease usually occurs in skin; also skeleton, bone marrow, GI tract, CNS
- Mast Cell Mediators
- Histamine and metabolites
- Tryptase
- Prostaglandin D2
- Heparin
- No humans with absence of mast cells have been reported
| Curr Cancer Drug Targets. 2004 May;4(3):267-83. |
|
Leukotriene A4 hydrolase
as a target for cancer prevention and therapy.
Chen X, Wang S, Wu N, Yang CS.
Susan Lehman Cullman Laboratory for Cancer Research, Department of Chemical
Biology, Ernest Mario School of Pharmacy, Rutgers, the State University of New Jersey, 164 Frelinghuysen Road, Piscataway,
New Jersey 08854, USA. xiaochen@rci.rutgers.edu
Leukotriene A4 hydrolase (LTA4H) is a bifunctional zinc enzyme with
the activities of epoxide hydrolase and aminopeptidase. As an epoxide hydrolase, LTA4H catalyzes the hydrolysis of the epoxide
LTA4 to the diol, leukotriene B4 (LTB4), which mainly functions as a chemoattractant and an activator of inflammatory cells.
As an aminopeptidase, LTA4H may process peptides related to inflammation and host defense. In a chronic inflammation-associated
animal model of esophageal adenocarcinoma, we have shown that LTA4H was overexpressed in tumor as compared to normal tissues.
Bestatin, an LTA4H inhibitor, suppresses tumorigenesis in this animal model. Since LTA4H has long been regarded as an anti-inflammatory
target, we propose LTA4H as a target for prevention and therapy of cancers, especially those associated with chronic inflammation.
Here we review the gene structure, expression, regulation and functions of LTA4H, as well as its involvement in carcinogenesis.
We believe LTA4H/LTB4 may play an important role in chronic inflammation associated carcinogenesis by at least two mechanisms:
a) the inflammation-augmenting effect on inflammatory cells through positive feedback mediated by its receptors and downstream
signaling molecules; and b) the autocrine (Secretion of a substance, such as a growth factor, that stimulates the secretory cell itself. One route to independence of growth control is by autocrine growth factor production. ) growth-stimulatory effect of LTB4 produced by epithelial cells, and the paracrine ( Form of signalling in which
the target cell is close to the signal releasing cell. Neurotransmitters and neurohormones are usually considered to fall into this category. ) growth-stimulatory effect of LTB4 produced by inflammatory cells, on precancerous and cancer
cells. Based on our present knowledge, inhibitors of LTA4H or antagonists of LTB4 receptors may be used alone or in combination
with other agents (e.g., cyclooxygenase 2 inhibitors) in cancer prevention and treatment trials to test their effectiveness.
| Gene. 1995 Aug 19;161(2):249-51. |
|

Amino-acid sequence and tissue
distribution of guinea-pig leukotriene A4 hydrolase.
Minami M,
Mutoh H, Ohishi N, Honda Z, Bito H, Shimizu T.
Department of Biochemistry, Faculty of Medicine, University of Tokyo,
Japan.
The guinea-pig leukotriene A4 hydrolase (LTA4H)-encoding cDNA was isolated from a guinea-pig lung cDNA
library by cross-hybridization using a human probe. The deduced amino acid (aa) sequence consists of 611 aa (68 756 Da) and
contains all twelve internal peptide and N-terminal sequences determined from the purified enzyme from guinea-pig intestine.
The aa identity of the guinea-pig enzyme with its human, mouse and rat counterparts was 92.9, 90.5 and 90.4%, respectively.
The previously characterized zinc-binding motif and a putative active site were highly conserved, supporting the aminopeptidase
activity described for this enzyme. RNA blot analysis demonstrated ubiquitous (Present everywhere. The small protein called ubiquitin was so-named because it is present in all types of cells and its amino acid sequence is identical in all creatures from insects to humans.) expression of the LTA4H mRNA.
Translocation of 5-lipoxygenase to the membrane in human
leukocytes challenged with ionophore A23187
reference 25 in co-localization article/Brock
CA Rouzer and S Kargman
Merck Frosst Canada Inc., Pointe Claire-Dorval, Quebec, Canada.
Challenge of human peripheral blood leukocytes with ionophore A23187 resulted in leukotriene (LT)
synthesis, a decrease in total cellular 5- lipoxygenase activity, and a change in the subcellular localization
of the enzyme. In homogenates from control cells, greater than 90% of the 5-lipoxygenase activity and
protein was localized in the cytosol (100,000 X g supernatant=The soluble liquid fraction of a sample after centrifugation or precipitation of insoluble solids). Ionophore challenge (2 microM) resulted in a loss of approximately 55% of the enzymatic activity and 35% of
the enzyme protein from the cytosol. Concomitantly, there was an accumulation of inactive 5-lipoxygenase
in the membrane (100,000 X g pellets) which accounted for at least 45% of the lost cytosolic protein. There was
a good correlation between the quantities of LT synthesized and 5-lipoxygenase recovered in the membrane
over an ionophore concentration range of 0.1-6 microM. The time course of the membrane association was similar
to that of LT synthesis. Furthermore, although the pellet-associated enzyme recovered from ionophore-treated
leukocytes was inactive, an irreversible, Ca2+-dependent membrane association of active 5-lipoxygenase could be
demonstrated in cell-free systems. To determine whether ionophore treatment induced proteolytic degradation
of 5-lipoxygenase, the total activity and protein content of 10,000 X g supernatants from control and ionophore-treated
cells were examined. These supernatants, which included both cytosolic and membrane-associated enzyme, showed a
35% loss of 5-lipoxygenase activity but only an 8% loss of enzyme protein as a result of ionophore challenge
(2 microM). Therefore, the majority of the loss of 5- lipoxygenase activity was most likely due to suicide inactivation
during the LT synthesis, rather than to proteolytic degradation. Together these results are consistent
with the hypothesis that ionophore treatment results in a Ca2+-dependent translocation of 5- lipoxygenase from
the cytosol to a membrane-bound site, that the membrane-associated enzyme is preferentially utilized
for LT synthesis, and that it is consequently inactivated. Thus, membrane translocation of 5-lipoxygenase may be
an important initial step in the chain of events leading to full activation of this enzyme in the intact
leukocyte.
LTA4 serves as the precursor for the potent
chemotactic factor, LTB4 as well as for the smooth muscle
contracting agonists LTC4, LTD4 and LTE4. These products
are secreted by leukocytes after exposure to various immunologic
and inflammatory stimuli, and their biological activities
suggest that they may play a role in immediate hypersensitivity
reactions and inflammation (4). Therefore, the regulation
of the 5-lipoxygenase may be of great importance to
the pathophysiology of these processes.
Calcium ionophore:
Ionophores are compounds that increase the permeability of cellular membrane barriers to ions by functioning
as mobile ion carriers or channel formers. They contain hydrophobic regions conferring lipid solubility and hydrophilic ion-binding
regions which delocalise the charge of the ion to shield it from the hydrophobic regions of the membrane lipid bilayer.
Calcium ionophoreA23187 is an artificial mobile iron carrier that normally acts as an ion-exchange shuttle molecule transporting one divalent
calcium ion into the cell in exchange of two H +. As intracellular calcium levels can be monitored by a variety of fluorescent probes use of A23187 provides information about the involvement of elevated levels of cytosolic free calcium (and indirect information
about associated secondary messenger systems) following cytokine receptor mediated signal transduction processes. A postulated
participation of calcium in the process under study can be confirmed by employing calcium-specific chelators such as EGTA
to produce very low intracellular calcium levels which should then block the response.
A23187 can cause cell activation , differentiation, or proliferation and thus mimics cellular processes normally observed in response to cytokines . It can be used, therefore, to probe functional capacities of cells and to dissect complex processes into a
series of discrete stages at the molecular level. Responses can include production and release cytokines , expression of an ERG (Early response gene ), an Oncogene , differentiation antigens (see also: CD antigens ), and intracellular adhesion molecules, cell death by apoptosis , progression through the Cell cycle , or inhibition of any of these processes.
Simultaneous treatment of cells with calcium ionophores and other agents (cytokines , drugs, hormones) can be used to investigate whether any of these agents affect (enhance or reverse) any of
the elicited responses.
For other agents used to dissect signal transduction pathways mediated by cytokines see: Bryostatins , Calphostin C , Genistein , H8 , Herbimycin A , K-252a , Lavendustin A , Phorbol esters , Okadaic acid , Staurosporine , Suramin , Tyrphostins , Vanadate .
is widely used for DNA/RNA detection/isolation due to the extremely high affinity of
the biotin-streptavidin interaction (association constant 1015/M). Biotin moieties can be incorporated within
an oligo on any place and in any number. We have long and super-long tethering arms covalently attached to biotin for improved binding kinetics, increased binding capacity of large DNA fragments, and for accessibility to enzymatic
events occurring at the solid-phase surface.
Blocking kit reagents may be used to block nonspecific binding of Biotin/Avidin System reagents:
http://www.vectorlabs.com/Protocols/SP2001.pdf
Principle:
Some tissues may bind avidin, biotinylated horseradish peroxidase or other Biotin/Avidin System
components
without prior addition of biotinylated antibody. This binding may be due to endogenous biotin or
biotin-binding
proteins, lectins, or nonspecific binding substances present in the section. If a high background
is present using the
ABC reagents (or other avidin conjugate) in the absence of biotinylated secondary antibody, pre-treatment
of the
tissue with avidin, followed by biotin (to block the remaining biotin binding sites on the avidin),
may be required.
The blocking kit consists of an Avidin D solution and a biotin solution. Pre-treatment of the section
with the
Avidin D solution should always be followed by incubation with the biotin solution. The Avidin
D and biotin
solutions should be used directly as supplied.
Suggested Protocol for Tissue Sections:
After incubation with normal serum, incubate section with Avidin D solution for 15 minutes. Rinse
briefly
with buffer, then incubate for 15 minutes with the biotin solution. These steps should be performed
prior to
the addition of primary antibody or lectin.
Biotin-(Strept)avidin Systems:
http://www.vectorlabs.com/products.asp?catID=28&locID=0
| Previous Methods |
| Before we offered the Biotin-Avidin System, which now also includes the use of streptavidin,
researchers used directly-labeled, enzyme-tagged primary and secondary antibodies for detection of tissues, blots, and microtiter
plates (Figs. 1 and 2). These direct/indirect methods were followed by the PAP method (Fig. 3), which provided some amplified
signal. |
| The Biotin-Avidin System |
| When we introduced the Biotin-Avidin System a substantial amplification over the earlier
methods was achieved. Avidin is an egg-white derived glycoprotein with an extraordinarily high affinity (affinity constant
> 1015 M-1) for biotin. Streptavidin is similar in properties to avidin but has a lower affinity
for biotin. Many biotin molecules can be coupled to a protein, enabling the biotinylated protein to bind more than one molecule
of avidin. If biotinylation is performed under gentle conditions, the biological activity of the protein can be preserved.
By covalently linking avidin with different ligands such as fluorochromes, enzymes or EM markers, what we have termed the
Biotin-Avidin System can be utilized to study a wide variety of biological structures and processes. The Biotin-Avidin System
has proven to be particularly useful in the detection and localization of antigens, glycoconjugates, and nucleic acids by
employing biotinylated antibodies, lectins, or nucleic acid probes. |
| Various Biotin-Avidin Methods |
| Over the years, several basic biotin-avidin techniques have evolved. One of the earliest
and currently least used has been termed the “sandwich” or “bridge” technique and relies on the multiple
biotin binding sites on avidin. Following the application of a biotinylated antibody, avidin is added, and then a biotinylated
enzyme or EM marker. (This technique is not shown.) |
| The next technique that evolved has been called the “covalent conjugate”
or “labeled avidin” (or “labeled streptavidin”) procedure (Fig. 4). Following the addition of a biotinylated
primary or secondary reagent, a covalent conjugate between (strept)avidin and an enzyme, fluorochrome, or EM marker is added.
|
| One example might be staining a histological section with monoclonal mouse primary
antibody directed toward a specific determinant on the cells. After the primary antibody is applied, biotinylated anti-mouse
IgG is added, followed by peroxidase conjugated avidin. |
| Development is accomplished by adding an appropriate substrate for peroxidase.
|
| The most recent and the most widely used technique is our patented(U.S. Patent No.
4,684,609) procedure called the “ABC” or “preformed complex” method. After application of a biotinylated
secondary or primary antibody, a preformed complex between avidin or streptavidin and a biotinylated enzyme is added (Figs.
5 and 6). This latest technique appears to be the most sensitive in many applications and is discussed more fully in the VECTASTAIN®
ABC section of this catalog. |
 |
 |
| Although each technique has its merit, it is now widely appreciated that the Biotin-Avidin
System provides the highest sensitivity in fluorescence and enzyme-based detection, versatility that allows easy interchange
or introduction of multiple markers, and the ability to localize or detect antigens which are difficult or impossible to see
or measure with other systems. |
| Advantages of the Biotin-Avidin System |
| The Biotin-Avidin System has several advantages over direct coupling of the marker
to an antibody, a lectin or a nucleic acid probe. |
|
The Biotin-Avidin System can improve sensitivity because of the potential for amplification
due to multiple site binding. |
|
Avidin can be prepared with high fluorochrome to protein ratios and avidin conjugates
are very stable. |
|
Only a single labeled conjugate, namely avidin or streptavidin, need be kept on hand
since it can be used with a variety of biotinylated lectins, antibodies or probes. |
|
Biotin-Avidin System reagents can overcome the problem of background fluorescence
sometimes encountered in the use of heavily fluorescein-labeled or rhodamine-labeled antibody. These conjugates are sometimes
“sticky” and adsorb nonspecifically to tissues, while fluorochrome-conjugated Avidin D does not. |
|
The extraordinarily high affinity between avidin or streptavidin and biotin assures
the user of a rapidly formed and stable complex between the (strept)avidin conjugate and the biotin-labeled protein.
|
|
Simultaneously localizing more than one antigen in the same tissue section can be
performed even with two or three primary antibodies from the same species. By using either separate enzyme systems, two different
substrates for the same enzyme, or assorted fluorochrome conjugates, more than one antigen can be localized in the same tissue
section. |
| Applications of the Biotin-Avidin System |
| Biotin-Avidin System reagents have been found to be superior substitutes for many
conventional, less sensitive methods. Just a few of these uses include: |
|
Immunohistochemical staining |
|
Introducing multiple labels into tissues |
|
Localizing hormone binding sites |
|
Flow Cytometry |
|
Nitrocellulose and nylon transfer blot detection |
|
In situ hybridization |
|
Radio-, enzyme-, and fluorescent immunoassays |
|
Neuronal tracing |
|
Genetic mapping |
|
Hybridoma screening |
|
Purification of cell surface antigens |
|
Coupling of antibodies and antigens to agarose |
|
Examination of membrane vesicle orientation |
| * |
adenocarcinoma:
<oncology, tumour> A form of cancer that involves cells from the lining of the walls of many different organs of the body. Breast cancer is a type of adenocarcinoma.
Esophageal adenocarcinoma is the faster growing cancer in the western world. Major risk factors for this cancer
are Gastroesophageal Reflux Disease (GERD) and Barrett's esophagus. http://heartburn.about.com/od/esophagealcancer/
esophagogastroduodenal anastomosis: anastomosing the duodenum to the gastroesophageal junction
columnar-lined esophagus:
columnar:1:of, relating to, resembling, or characterized by columns/ 2:of, relating to, being, or composed of tall narrow somewhat cylindrical or prismatic epithelial cells
lined: to place or form a line along
| Mutat Res. 2003 Feb-Mar;523-524:137-44. |
|

Mechanisms and
applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer.
Steele VE, Hawk
ET, Viner JL, Lubet RA.
Division of Cancer Prevention, National Cancer Institute, National Institutes of Health,
9000 Rockville Pike, Bethesda, MD 20892-7322, USA. vsly@nih.gov
Biological and chemical irritants can be the cause
of irritation in a variety of organ sites. It is becoming well understood that chronic irritation in any form can initiate
and accelerate the cancer process in these same organs. This understanding comes in part from the many epidemiologic studies
which point out that chronic inflammation correlates with increased risk of developing cancer in that organ which is affected.
One of the hallmarks of chronic irritation is the increased activity in the arachidonic acid pathway which provides many of
the necessary inflammatory biochemical mediators to this process. Arachidonic acid metabolism diverges down two main pathways,
the cyclooxygenase (COX) and the lipoxygenase (LOX) pathways. The COX pathway leads to prostaglandin and thromboxane production
and the LOX pathway leads to the leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs). These classes of inflammatory
molecules exert profound biological effects which enhance the development and progression of human cancers. A large number
of synthetic drugs and natural products have been discovered that block many of these key pathways. Much experimental evidence
in animals has shown that inhibition of the key enzymes which drive these pathways can, in fact, prevent, slow or reverse
the cancer process. The data are convincing in a number of organ sites including colon, breast, lung, bladder and skin. More
recently, double-blinded randomize clinical trials in humans have shown the prevention of colonic polyps by anti-inflammatory
agents. These studies have primarily used non-steroidal anti-inflammatory drugs (NSAIDS) which block the COX pathways. Recent
preclinical studies indicate that the LOX pathway also may be an important target for cancer prevention strategy. The expression
of high levels of these enzymes in cancerous tissues make them an obvious first target for cancer prevention strategies. As
newer more specific drugs are developed with few adverse effects this important prevention strategy may become a reality.
Clinical Cancer Research Vol. 10, 6703-6709, October 1, 2004
© 2004 American Association for Cancer Research
Experimental Therapeutics, Preclinical Pharmacology |
Overexpression of 5-Lipoxygenase in Rat and Human Esophageal
Adenocarcinoma and Inhibitory Effects of Zileuton and Celecoxib on Carcinogenesis Xiaoxin
Chen1, Su Wang1, Nan Wu1, Sandeep Sood1,
Peng Wang1, Zhe Jin1, David G. Beer2, Thomas
J. Giordano3, Yong Lin4, Wei-chung J. Shih4, Ronald
A. Lubet5 and Chung S. Yang1
1 Susan Lehman Cullman Laboratory for Cancer Research, Department of Chemical Biology, Ernest Mario
School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, New Jersey; 2 Section of General Thoracic
Surgery, Department of Surgery and 3 Department of Pathology, University of Michigan, Ann Arbor, Michigan; 4
Division of Biometrics, University of Medicine and Dentistry of New Jersey School of Public Health, New Brunswick, New Jersey;
and 5 Chemoprevention Branch, Division of Cancer Prevention, National Cancer Institute, Bethesda, Maryland
Purpose: Aberrant arachidonic acid (AA) metabolism, especially through the cyclooxygenase
(Cox) and 5-lipoxygenase (5-Lox) pathways, has been suggested to play an important role in the development
of esophageal adenocarcinoma (EAC). The purpose of this study was to investigate the expression of 5-Lox in
EAC of a rat model and in human samples as well as the chemopreventive effects of zileuton (a specific 5-Lox
inhibitor) and celecoxib (a specific Cox2 inhibitor) in the rat EAC model.
Experimental Design: 5-Lox expression in EAC of a rat esophagogastroduodenal anastomosis
model and of humans was examined with immunohistochemistry. A chemoprevention study was designed to test whether
zileuton and celecoxib could suppress aberrant AA metabolism and esophageal adenocarcinogenesis.
Results: With immunohistochemistry, we found that 5-Lox was overexpressed during esophageal
adenocarcinogenesis in our rat model and in humans. In the chemoprevention study, EAC incidence was
reduced in a dose-dependent manner from 68.8% (11 of 16) to 44.4% (8 of 18; P > 0.05) and 31.3% (5 of
16; P < 0.05) by 500 and 1,000 ppm zileuton, respectively, and to 33.3% (7 of 21; P
< 0.05) and 20% (3 of 15; P < 0.05) by 500 and 1,000 ppm celecoxib, respectively. With isobolographic
analysis, zileuton and celecoxib, both at a dose of 500 ppm, had an additive effect by reducing the
tumor incidence to 16.7% (3 of 18, P < 0.01). Leukotriene B4 and prostaglandin E2
levels in the esophageal tissues were also significantly reduced by zileuton and celecoxib.
Conclusions: This study clearly demonstrated that 5-Lox and Cox2 play important roles in
the development of EAC. Both zileuton and celecoxib had inhibitory effects on esophageal adenocarcinogenesis
through inhibition on their respective enzymes of AA metabolism.
Copyright © 2002 Elsevier Science Inc. All rights reserved.
Leukotriene A4 hydrolase
Jesper Z. Haeggström , ,  , a, Filippa Kulla, Peter C. Rudberga, Fredrik Tholandera and Marjolein M. G. M. Thunnissenb , a, Filippa Kulla, Peter C. Rudberga, Fredrik Tholandera and Marjolein M. G. M. Thunnissenb
a Department of Medical Biochemistry and Biophysics, Division of Chemistry II, Karolinska Institutet,
S-171 77, Stockholm, Sweden
b Department of Biochemistry, Arrhenius Laboratories A4, University of Stockholm, S-106 91, Stockholm,
Sweden
Available online 6 September 2002.
Abstract
The leukotrienes (LTs) are a family of lipid mediators involved in inflammation and allergy. Leukotriene B4
is a classical chemoattractant, which triggers adherence and aggregation of leukocytes to the endothelium at only nanomolar
concentrations. In addition, leukotriene B4 modulates immune responses, participates in the host-defense against
infections, and is a key mediator of PAF-induced lethal shock. Because of these powerful biological effects, leukotriene B4
is implicated in a variety of acute and chronic inflammatory diseases, e.g. nephritis, arthritis, dermatitis, and chronic
obstructive pulmonary disease. The final step in the biosynthesis of leukotriene B4 is catalyzed by leukotriene
A4 hydrolase, a unique bi-functional zinc metalloenzyme with an anion-dependent aminopeptidase activity. Here we
describe the most recent developments regarding our understanding of the structure, function, and catalytic mechanisms of
leukotriene A4 hydrolase.
Author Keywords: Leukotriene; Aminopeptidase; Inflammation; Crystal structure; Epoxide hydrolase

Copyright © 1998 Elsevier Science Ltd. All rights reserved
Molecules in focus
Leukotriene B4
S. W. Crooks* and R. A. Stockley
Department of Medicine, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH UK
Received 19 July 1997; accepted 19 September 1997. Available online 22 July 1998.
Abstract
Leukotriene B4 is a pro-inflammatory mediator synthesised in myeloid cells from arachidonic acid. Synthesis
is catalysed by 5-lipoxygenase and leukotriene A4 hydrolase and is increased by inflammatory mediators including endotoxin,
complement fragments, tumor necrosis factor and interleukins. A nuclear membrane protein, 5-lipoxygenase activating protein,
is an essential co-factor for 5-lipoxygenase. Leukotriene B4 induces recruitment and activation of neutrophils, monocytes
and eosinophils. It also stimulates the production of a number of proinflammatory cytokines and mediators indicating an ability
to augment and prolong tissue inflammation. Elevated levels of leukotriene B4 have been found in a number of inflammatory
diseases and levels are related to disease activity in some of these. Initial data from pharmacological inhibition studies
support a role for leukotriene B4 in the pathogenesis of neutrophil mediated tissue damage, and treatments which reduce its
production or block its effects may prove beneficial in neutrophil mediated inflammatory diseases.
Leukotriene A4-hydrolase expression
and leukotriene B4 levels in chronic inflammation of bacterial origin
Immunohistochemistry and reverse-phase
high-performance liquid chromatography analysis of oral mucosal epithelium
Virchows Archiv
Publisher: Springer-Verlag Heidelberg
ISSN: 0945-6317 (Paper) 1432-2307 (Online)
DOI: 10.1007/s00428-001-0597-2
Issue: Volume 440, Number 6
Date: June 2002
Pages: 627 - 634
Jörg Eberhard, Søren Jepsen, Markus Tiemann, Ralph Krause, Yahya Açil, Hans-Karl Albers
A1 Department of Operative Dentistry and Periodontology, University of Kiel, Arnold-Heller-Strasse
16, 24105 Kiel, Germany
A2 Department of Hematopathology and Lymph Node Registry, University of Kiel, Germany
A3 Department
of Oral and Maxillofacial Surgery, University of Kiel, Germany
Abstract:
Abstract. Chronic inflammation of the oral epithelium of bacterial origin is associated with elevated leukotriene
B4 (LTB4) levels. We investigated leukotriene A4 (LTA4)-hydrolase expression and
LTB4 levels in oral epithelium in relation to the clinical disease manifestation and immunohistopathology and LTA4-hydrolase
expression in cultured oral keratinocytes. In 11 patients, three different types of biopsy specimens of the oral mucosa tissues
were examined . Each sample was divided, and one-half was analysed using immunohistochemistry with antibodies to LTA4-hydrolase,
CD1a, CD3, CD19, macrophages/monocytes and granulocytes. The other half of the sample was homogenised and analysed using reverse-phase
high-performance liquid chromatography to determine LTB4 levels. We found strong LTA4-hydrolase expression
in basal cells of the oral epithelium from tissue samples that appeared clinically healthy; however, histologically a mild
chronic inflammation was observed. In contrast, patients with symptoms of an inflammation of the oral mucosa showed only weak
LTA4-hydrolase staining of the epithelial cell layers, but strong immunoreactivity in endothelial and invading
inflammatory cells. LTB4 levels were elevated in inflamed tissues compared with non-inflamed controls. Most significantly,
there was a strong association between the immunohistochemical detection of the enzyme, LTB4 levels, cellular infiltration
and the clinical disease manifestations. In vitro experiments indicated that LTA4-hydrolase expression may be induced
by bacterial contamination. This study suggests that LTA4-hydrolase expression and elevated LTB4 levels
in oral mucosal epithelium are integral parts of the induction and progression of chronic inflammatory reactions. Epithelial
cells may participate in early stages of inflammation as a source of LTB4.
| Curr Pharm Des. 2001 Feb;7(3):163-79. |
|
Inhibitors of leukotriene
A4 (LTA4) hydrolase as potential anti-inflammatory agents.
Penning TD.
Pharmacia, Corp.,
4901 Searle Parkway, Skokie, IL 60077, USA. thomas.d.penning@monsanto.com
Leukotriene A4 (LTA4) hydrolase is a zinc-containing
enzyme which stereospecifically catalyzes the hydrolysis of the epoxide LTA4 to the diol leukotriene B4 (LTB4). There is substantial
evidence that LTB4 plays a significant role in the amplification of many inflammatory disease states. Therapeutic agents which
selectively inhibit LTA4 hydrolase would block the formation of LTB4 and thus be potentially useful for the treatment of inflammation.
Numerous inhibitors of LTA4 hydrolase have been reported over the past 15-20 years. Several early inhibitors were based on
the structure of the natural substrate, LTA4. Later approaches utilized known inhibitors of related zinc-containing metalloproteinases
and led to the identification of captopril, bestatin and kelatorphan as potent inhibitors of LTA4 hydrolase. This led to the
design of a number of peptide and non-peptide analogs which contained potential zinc-chelating moieties, including thiols,
hydroxamates and norstatines. A more recent series of non-peptidic, non-zinc chelating inhibitors of LTA4 hydrolase has been
reported. This work led to the identification of several novel classes of analogs, including imidazopyridines, amidines and
cyclic and acyclic amino acid derivatives, ultimately resulting in the identification of two potential clinical candidates
SC-56938 and SC-57461A.
Copyright © 1994 Published by Elsevier Science Inc.
Modulation of pulmonary leukotriene formation and perfusion
pressure by bestatin, an inhibitor of leukotriene A4 hydrolase
Danny T. Muskardin, Norbert F. Voelkel and F. A. Fitzpatrick
Departments of Pharmacology and Medicine, University of Colorado Health Sciences Center, Denver, CO
80262, U.S.A.
Received 2 September 1993; accepted 23 February 1994. Available online 8 November 2002.
Abstract
We investigated the effects of bestatin, a prototype leukotriene A4 (LTA4 hydrolase
inhibitor, on leukotriene (LT) formation and pulmonary artery perfusion pressure (Ppa) in isolated, perfused rat
lungs. In lung parenchymal strips stimulated with a 10  M concentration of the Ca2+ ionophore A23187, bestatin inhibited LTB4 formation with an IC50
= 10.4 ± 3.0 M concentration of the Ca2+ ionophore A23187, bestatin inhibited LTB4 formation with an IC50
= 10.4 ± 3.0  M (mean ± SD, N = 4). It did not alter cysteinyl LT formation, confirming that it inhibited LTA4 hydrolase
selectively, without inhibiting phospholipase, 5-lipoxygenase, or LTC4 synthase. In isolated, perfused lungs stimulated
with 10 M (mean ± SD, N = 4). It did not alter cysteinyl LT formation, confirming that it inhibited LTA4 hydrolase
selectively, without inhibiting phospholipase, 5-lipoxygenase, or LTC4 synthase. In isolated, perfused lungs stimulated
with 10  M A23187, 300 M A23187, 300  M bestatin inhibited LTB4 release by 72.2 ± 10.6% (mean ± SEM, N = 6, P < 0.01) but had no significant effect
on LTE4 formation (P > 0.5). In these perfused lungs, bestatin did not alter the change in Ppa following
stimulation with A23187. This effect is consistent with the insubstantial re-direction of LTA4 toward formation
of vasospactic cysteinyl LTs. Separate experiments used lungs from rats treated with lipopolysaccharide endotoxin in vivo,
prior to isolation, perfusion, and stimulation with 5 M bestatin inhibited LTB4 release by 72.2 ± 10.6% (mean ± SEM, N = 6, P < 0.01) but had no significant effect
on LTE4 formation (P > 0.5). In these perfused lungs, bestatin did not alter the change in Ppa following
stimulation with A23187. This effect is consistent with the insubstantial re-direction of LTA4 toward formation
of vasospactic cysteinyl LTs. Separate experiments used lungs from rats treated with lipopolysaccharide endotoxin in vivo,
prior to isolation, perfusion, and stimulation with 5  m formyl-methionyl-leucyl-phenylalanine, in vitro. In these inflamed lungs, 750 m formyl-methionyl-leucyl-phenylalanine, in vitro. In these inflamed lungs, 750  M bestatin inhibited LTB4 formation (P < 0.05) and increased LTE4 formation (P < 0.05), compatible
with selective inhibition of LTA4 hydrolase. The re-direction of LTA4 metabolism toward formation of
cysteinyl LTs by inflamed, perfused lungs did not cause an increase in Ppa. M bestatin inhibited LTB4 formation (P < 0.05) and increased LTE4 formation (P < 0.05), compatible
with selective inhibition of LTA4 hydrolase. The re-direction of LTA4 metabolism toward formation of
cysteinyl LTs by inflamed, perfused lungs did not cause an increase in Ppa.
- Removal of an entire structure (such as an eyeball or tumor), without rupture, as one
shells the kernel of a nut.
- Removal or destruction of the nucleus of a cell.
enucleation (enu·cle·a·tion) (e-noo²kle-a¢sh[schwa]n)
[L. e out + nucleus kernel] the removal of an organ, of a tumor, or of another body in such a way
that it comes out clean and whole, like a nut from its shell. Used in connection with the eye, it denotes removal of the eyeball
after the eye muscles and optic nerve have been severed.
ficoll
This biochemically inert sucrose polymer is used as athickening additive in solutions and gradients
ficoll gradient
A density gradient of ficoll (synthetic sucrose polymer) in solution, where concentration of the ficoll varies continuously through the solution. It is often used to separate different types of cells from each other during the process of sedimentation.
Ficoll-Hypaque technique
A density-gradient centrifugation technique for separating lymphocytes from other formed elements in the blood; the sample is layered onto a Ficoll-sodium metrizoate gradient of specific density; following centrifugation, lymphocytes are collected from the plasma-Ficoll interface
ficoll http://www.med.upenn.edu/bmcrc/immune/Ficcoll_info.pdf
Ficoll ™ PM 70 and Ficoll
PM 400 are high molecular weight
sucrose-polymers formed by copolymerization of sucrose
with epichlorohydrin. The molecules are highly branched and
the high content of hydroxyl groups leads to very good
solubility in aqueous media. Ficoll PM 70 and PM 400 are
supplied as spray-dried powders. Ficoll behaves as an ideal
neutral sphere and has been proposed as the molecule of
choice for studying pore size distribution and the permeability
of membranes. Ficoll PM 70 and Ficoll PM 400 have
analogous structures, but differ in molecular weight, and are
therefore appropriate for different applications.
Stability
The stability of Ficoll is chiefly determined by the glycosidic
bonds in the sucrose residues. Ficoll does not contain any
ionized groups, so the structure does not react under
physiological conditions. It is stable in alkaline and neutral
solutions, but is rapidly hydrolyzed in solution at pH 3,
especially at elevated temperature. Ficoll can be sterilized by
autoclaving at 110 ºC for 30 minutes in neutral solutions.
Strong oxidizing and reducing agents should be avoided.
Shipping and storage are at ambient temperatures.
Chemical and physical properties
Ficoll is provided as a dry powder and is extremely
hydrophilic. Solutions are best prepared by slowly stirring
Ficoll into aqueous buffer. Gentle heating may be required for
complete solubilization.
Centrifugation
In centrifugation methods, the density and viscosity of the
medium are adjusted to allow particle sedimentation with a
convenient speed. With sucrose, the high osmotic pressure,
which results from the concentrations used, often damages
the cells. If, instead, you add a high molecular weight
polymer such as Ficoll, you obtain the required density
without significantly increasing the osmotic pressure. This
preserves cells intact and retains their viability. Ficoll is
therefore preferred to sucrose for forming density gradients,
and is primarily used in this way for the routine separation of
cells (10, 11, 12).
Ficoll PM 400 can be used for gradient centrifugation in all
types of centrifuge rotors and for separation at unit gravity.
For centrifugation, both discontinuous and continuous
gradients are possible. Discontinuous gradients offer two
main advantages: First, the abrupt changes in Ficoll PM 400
density mean that isolated cells are found in sharp bands at
the interface between layers of different density. This allows
for easy removal of the purified sample with a pipette.
Second, cells with great differences in density can easily be
isolated with as few as two density layers. This is achieved by
choosing densities that will prevent one or more type of cell
from entering the lower phase, banding these cell types at the
interface. To estimate the densities required for a particular
application, refer to Table 1.
Discontinuous gradients are established as follows:
1. Using Table 1 as a guide, dissolve Ficoll PM 400 in buffer
or isotonic (0.25 M) sucrose solution at various
concentrations (generally differing by 5-10% w/v), which
should separate the cells of interest. Most cells and organelles
have a buoyant density between 1.0 and 1.2 g/ml in
Ficoll PM 400. Often, a simple two-layer gradient is sufficient.
You may store these fractions in a refrigerator, but ensure
that they reach room temperature before use.
2. In normal centrifuge tubes, make layers (approx. 1 cm deep)
of decreasing density with the most dense solution at the bottom.
3. Keep the tubes at room temperature for a few hours to
allow diffusion across the interfaces, and thereby even out the
sharp borders between fractions.
4. Layer the suspension to be fractionated carefully on top.
Stir the sample and upper Ficoll layer gently with a glass rod
to eliminate the interface between them before centrifugation.
During centrifugation, particles collect either in or between
the various Ficoll layers, depending on the density of the
layers. The cells/organelles collect at a lower density than on
sucrose gradients of equivalent concentration, as Ficoll does
not penetrate cell membranes. After centrifugation, pipette
off the various phases, and remove the Ficoll from the
required fraction by repeatedly diluting with buffer, and
centrifuging to sediment the particles. Residual amounts of
Ficoll PM 400 in the sample can be estimated with the
anthrone reaction (1).
|
La centrifugation est une méthode couramment utilisée en biochimie pour séparer ou analyser des fractions ou des structures
cellulaires, des macromolécules, etc.
On peut accentuer ou raffiner les méthodes de séparation en faisant cette centrifugation dans un gradient de concentration.
En effet, un des facteurs qui influence la vitesse de sédimentation est la différence entre la densité de la particule et
celle du solvant. On peut donc moduler cette vitesse en faisant varier de façon continue ou par étape (discontinue) cette
différence de densité en créant un gradient de concentration.
Si la densité de la particule est plus grande que celle du milieu, elle sédimentera. Plus la différence de densité est
grande plus la sédimentation est rapide. S'il n'y a aucune différence de densité, il n'y aura aucune sédimentation, quelle
que soit l'accélération. Si la particule est moins dense que celle du milieu, celle-ci s'élèvera dans le tube jusqu'à atteindre
un niveau de densité égal à la sienne ou, le cas échéant, jusqu'à flotter à la surface.
|
|
|
|
MÉTHODES ET APPAREILLAGE
Fabrication des gradients
Les variations de densités sont obtenues en faisant varier la concentration d'un produit chimique dans la solution. Divers
produits peuvent être utilisés pour faire ces gradients. Ils doivent être très solubles en solution aqueuse, ce qui permet
d'obtenir des densités suffisantes. On recherche aussi des produits qui sont relativement inertes, peu coûteux, faciles à
manipuler, non toxiques, etc. Évidemment aucun produit ne réunit toutes ces qualités et on doit choisir en tenant compte des
contraintes expérimentales
Le saccharose ("sucrose") est très souvent employé. Il permet d'atteindre des densités assez élevées, de l'ordre
de 1.3 g/mL avec du saccharose 2.5 M. Ce produit a l'avantage d'être peu coûteux, électriquement neutre et plutôt inerte pour
la plupart des fractions cellulaires. Son principal défaut est sa viscosité à forte concentration, ce qui rend son utilisation
plus difficile. Il est aussi à déconseiller si la pression osmotique est un facteur important, par exemple dans l'isolement
de cellules entières. |
http://www.chimie-biochimie.umoncton.ca/bch/dg/siitub/centgradient.html
Au début de la centrifugation, le mélange contenant la substance a isoler est déposée à la surface
du liquide. Au cours de la centrifugation, la molécule descend en fonction de sa densité. La vitesse de sédimentation est
exprimée dans une unité indépendante des densitées du milieu et de l'accélération : le Svedberg (S). Plus la valeur en Svedberg
est élevée, plus elle arrivera vite au fond du tube. A la fin de la centrifugation, le tube contient deux phases distinctes
: un dépot plus ou moins solide au fond du tube qui correspond au molécules ayant reussi à sédimenter jusqu'au bout et appelé
culot, un phase liquide qui contient toute les molécules n'ayant pas atteind le fond du tube, le surnageant. Selon les cas,
la molécules désirée est dans le culot, le surnageant ou repartie entre les deux (dans ce dernier cas, cela signifie que les
paramêtres de centrifugation ont été mal choisis).
http://webiologie.free.fr/techniques/purification/centrifugation.html
cytologie methodes:
http://www.fmed.ulaval.ca/bio-11134/Cytol-RP-Notes-p1.pdf
cy·to·plast The intact cytoplasm
of a single cell.
cytochalasin
A group of fungal metabolites that inhibit the addition of G actin to a nucleation site and therefore perturb labile microfilament arrays. Cytochalasin B inhibits at around 1 microgram/ml but at about 5 _g/ml begins to inhibit glucose transport. Cytochalasin D affects only the microfilament system and is therefore preferable.
alpha tubulin marker :
Tubulin is the protein that polymerizes into long chains or filaments that form microtubules, hollow fibers which serve
as a skeletal system for living cells. Microtubules have the ability to shift through various formations which is what enables
a cell to undergo mitosis or to regulate intracellular transport. The formation-shifting of microtubules is made possible
by the flexibility of tubulin which is why scientists have sought to understand the protein's atomic structure since its discovery
in the 1950s.
mouse monoclonal anti– -tubulin antibody (A11126) in combination with Alexa Fluor 546 goat anti–mouse IgG antibody -tubulin antibody (A11126) in combination with Alexa Fluor 546 goat anti–mouse IgG antibody
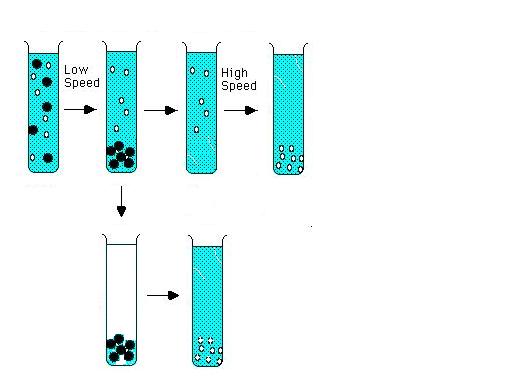
Moving boundary/Zone Centrifugation :
http://ntri.tamuk.edu/centrifuge/centrifugation.html
A third method of defining centrifugation is by the way the samples are applied to the centrifuge tube. In
moving boundary (or differential centrifugation), the entire tube is filled with sample and centrifuged. Through centrifugation,
one obtains a separation of two particles but any particle in the mixture may end up in the supernatant or in the pellet or
it may be distributed in both fractions, depending upon it size, shape, density, and conditions of centrifugation. The pellet
is a mixture of all of the sedimented components, and it is contaminated with whatever unsedimented particles were in the
bottom of the tube initially. The only component which is purified is the slowest sedimenting one, but its yield is often
very low. The two fractions are recovered by decanting the supernatant solution from the pellet. The supernatant can be recentrifuged
at higher speed to obtain further purification, with the formation of a new pellet and supernatant

In rate zonal centrifugation, the sample is applied in a thin zone at the top of the centrifuge tube on a density gradient.
Under centrifugal force, the particles will begin sedimenting through the gradient in separate zones according to their size
shape and density. The run must be terminated before any of the separated particles reach the bottom of the tube.
Zone Velocity Centrifugation:
http://bioweb.wku.edu/courses/Biol502/centrifugation.html
A method that results in yields greater than differential centrifugation and allows greater resolution of
all particles sizes is zone velocity centrifugation.  A sample is layered on top of a shallow density gradient and then centrifuged. Each particle size will migrate as a zone or
band at a characteristic velocity. If the velocities of the particles are sufficiently different then the zones of the particles
will resolve. The gradient through which the particles are centrifuged is used to stablilze the zones during recovery and
helps prevent mixing of resolved zones. The density of the gradient material should be less than the components being sedimented. A sample is layered on top of a shallow density gradient and then centrifuged. Each particle size will migrate as a zone or
band at a characteristic velocity. If the velocities of the particles are sufficiently different then the zones of the particles
will resolve. The gradient through which the particles are centrifuged is used to stablilze the zones during recovery and
helps prevent mixing of resolved zones. The density of the gradient material should be less than the components being sedimented.
Horseradish peroxidase
is a heme protein found in the roots of the horseradish and has a molecular weight of about 40,000
daltons. we see in the microscope a reaction product produced by incubation with an appropriate substrate and hydrogen peroxide.
In consequence, a relatively small number of enzyme molecules can create an easily visualized precipitate. Interestingly,
the sequence of reactions involved is similar to the reactions that produce melanins.
horseradish (horse·rad·ish) (h[omacr]rs¢rad-ish) 1. Armoracia lapathifolia. 2. any of several other plants of the family Cruciferae resembling A. lapathifolia. 3. the
pungent root of one of these plants, used as a condiment and appetite stimulant; in the past it was used as a rubefacient
and plaster like mustard, because it contains sinigrin.
horseradish peroxidase (horse·rad·ish per·ox·i·dase) (hors¢rad-ish
p[schwa]r-ok¢s[ibreve]-d[amacr]s²) peroxidase isolated from horseradish
(Armoracia lapathifolia); used as a reagent in biochemical assays. Abbreviated HRP .
The western blotting technique for the immunochemical detection and analysis
of proteins can be performed with radioisotopic, colorimetric, fluorescent, and chemiluminescent modes
of detection 1. The scope and flexibility of the fluorescence detection modes are now significantly enhanced
by the addition of a specific substrate for peroxidase-based western blots that allows for both chemiluminescent and fluorescent detection. The new Amersham Pharmacia Biotech ECL Plus substrate
used in the detection of horseradish peroxidase (HRP)-based westerns is compatible with direct
image analysis using the Molecular Dynamics Storm gel and blot imaging system. Storm system imaging of
western blots developed with ECL Plus results in detection limits that match those
obtained with film-based exposures and may complement, or offer advantages to, the previously described
ECF chemifluorescence approach2. Storm system image analysis of western blots using ECL Plus can be
applied to current HRPdetection protocols for fast and sensitive protein immune detection. http://www.link.med.ed.ac.uk/ELEGI/downloads/ECL%20plus.pdf
The chemiluminescent signal was captured on Hyperfilm™ (Amersham Pharmacia
Biotech) using a 30 sec exposure for optimal contrast.

http://www4.amershambiosciences.com/aptrix/upp01077.nsf/Content/Products?OpenDocument&parentid=577914&moduleid=46852#content
Speed and Sensitivity
Lumigen PS-atto is the newest substrate for the chemiluminescent detection
of HRP conjugates. Reaction of the substrate with an HRP label rapidly generates sustained high-intensity luminescence for
maximum detection sensitivity in solution assays. Lumigen PS-atto replaces Lumigen PS-1 for solution applications using peroxidase
detection.

Sites of Leukotriene Biosynthesis
http://www.jfmed.uniba.sk/patfyz/separatky/Role_of_Leukotrienes_in_Inflammation/t.html
The locations at which the leukotrienes are synthesized are determined by the cellular distribution of the enzymes
controlling each stage of the biosynthetic pathway. Because 5-lipoxygenase is only found in cells of myeloid lineage, the
synthesis of LTA4 is limited to these cells [17]. However, the enzymes determining the next step in the arachidonic acid cascade, either to LTB4 or
to the sulfidopeptide leukotrienes, are more widely distributed; thus, metabolism of LTA4 may occur in an equally
wide range of cell types. The export of LTA4 from cells that can actively synthesize it enables a much broader
range of cells to act as leukotriene secretors.

Figure 2. Model of cellular leukotriene biosynthesis. Stimulation of the cell leads to mobilization
of Ca2+, which triggers activation and translocation of cytosolic phospholipase A2 (cPLA2)
and 5-lipoxygenase (5-LO) to the nuclear envelope. Together with five lipoxygenase-activating protein (FLAP) these enzymes
constitute a biosynthetic complex that produces LTA4 for further biosynthesis of LTB4 and LTC4
via the soluble LTA4 hydrolase and membrane-bound LTC4 synthase, respectively. http://ajrccm.atsjournals.org/cgi/content/full/161/2/S1/S25
NOUVEAU REACTIF CHIMILUMINESCENT,
La luminescence est le phénomène par lequel certaines molécules portées à un état excité retournent
à l'état fondamental en restituant une partie de l'énergie sous forme d'émission de lumière. On distingue plusieurs types
de luminescence selon la source d'énergie impliquée dans le processus d'excitation.
Lorsque l'énergie qui permet aux molécules d'atteindre l'état excité provient d'une réaction
chimique, il s'agit du phénomène de chimiluminescence. La bioluminescence, qui est le phénomène d'émission de lumière observé
chez certains organismes vivants, peut être considérée comme un cas particulier de chimiluminescence pour lequel une protéine,
le plus souvent enzymatique, est impliquée dans la réaction génératrice de lumière.
La chimiluminescence est à l'origine de signaux utilisables en immunoanalyse, soit pour un dosage,
soit pour une recherche qualitative. Deux réactions d'émission de lumière sont particulièrement exploitées dans ce domaine
:
- la chimiluminescence des 1,2-dioxétanes,
- la chimiluminescence du luminol.
REACTION DE CHIMILUMINESCENCE DES 1,2-DIOXETANES.
Les 1,2-dioxétanes utilisés pour les mesures par luminescence sont des composés stables à température
ambiante et qui, sous l'action de la chaleur, se décomposent en deux produits carbonylés dont l'un est à l'état excité et
est donc susceptible d'émettre de la lumière. La stabilité des dioxétanes à température ambiante dépend de la nature des substituants
présents sur l'hétérocyclobutane;
Il est possible de synthétiser des dérivés stables non luminescents qui, sous l'action d'un
enzyme, produiront un intermédiaire instable se décomposant en émettant de la lumière. C'est le cas de l'AMPPD (3-(2'-spiroadamantane)-4-méthoxy-4-(3"-phosphoryloxy)phényl-1,2-dioxétane).
L'enzyme déclenchant est la phosphatase alcaline. L'hydrolyse enzymatique de cet ester phosphorique génére de l'AMPD-, instable,
qui se scinde en deux produits dont l'un, l'anion méthyl m-oxybenzoate émet de la lumière .
Un conjugué marqué par la phosphatase alcaline peut ainsi être détecté par cette méthode. L'addition
de micelles constituées de molécules fluorescentes et de bromure de cétyltriméthylammonium permet d'amplifier le signal luminescent
produit au cours de cette réaction.
REACTION DE CHIMILUMINESCENCE DU LUMINOL
En milieu alcalin, l'oxydation du luminol (5-amino-2,3-dihydrophthalazine-1,4-dione) produit
une émission lumineuse. L'agent oxydant le plus utilisé est le peroxyde d'hydrogène. Cette réaction de chimiluminescence peut
être catalysée par la peroxydase de raifort et la quantité de lumière émise est proportionnelle à la quantité de catalyseur
si les substrats de la réaction sont en excès.
http://www.gazettelabo.fr/2002archives/pratic/1996/5reactif.htm
|